 A.F. Maarten Altelaar, Ioana M. Taban, Liam A. McDonnell, Peter D.E.M. Verhaert, Robert P.J. de Lange, Roger A.H. Adan, Wolter J. Mooi, Ron M.A. Heeren and Sander R. Piersma
A.F. Maarten Altelaar, Ioana M. Taban, Liam A. McDonnell, Peter D.E.M. Verhaert, Robert P.J. de Lange, Roger A.H. Adan, Wolter J. Mooi, Ron M.A. Heeren and Sander R. Piersma
Int. J. Mass Spectrom. 2007, 260, pp. 203-211
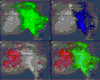
Matrix assisted laser desorption/ionization (MALDI) imaging mass spectrometry (IMS) has been used to determine peptide distributions directly from rat, mouse and human pituitary tissue sections. Since these organs are small (102–103 μm) the spatial resolution of IMS is a key issue in molecular imaging of pituitary tissue sections. Here we show that high-resolution IMS allows localization of neuropeptide distributions within different cell clusters of a single organ of a pituitary tissue section. The sample preparation protocol does not result in analyte redistribution and is therefore applicable to IMS experiments at cellular length scales. The stigmatic imaging mass spectrometer used in this study produces selected-ion-count images with pixel sizes of 500 nm and a resolving power of 4 μm, yielding superior spatial detail compared to images obtained in microprobe imaging experiments. Furthermore, we show that with imaging mass spectrometry a distinction can be made between different mammalian tissue sections based on differences in the amino acid sequence of neuropeptides with the same function. This example demonstrates the power of IMS for label-free molecular imaging at relevant biological length scales.
No responses yet